Personalized medicine is now one step closer to consumers. Thanks to the tiny, implantable sensors that could give an early warning of a person’s developing health problems – far before symptoms start to surface.
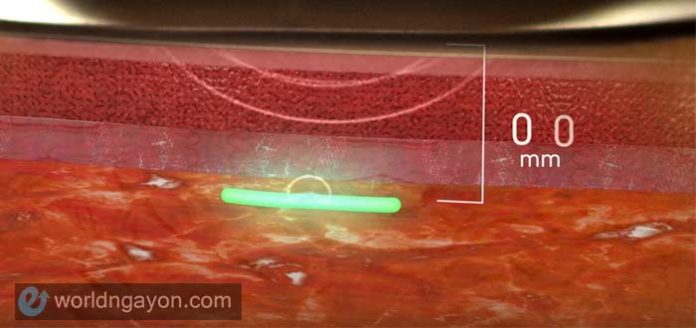
This implantable device uses bio-integrated sensors that cannot be detected as foreign object by the body, a drawback to the previously released implantable devices currently available in the market.
“Other implantable sensors currently on the market have a significant drawback, as they often provoke a ‘foreign body’ immune response that coats the sensor with inflammatory cells or scar tissue,” said Natalie Wisniewski, a researcher who presented the study at the 255th National Meeting & Exposition of the American Chemical Society (ACS) on 18-22 March 2018 at New Orleans Convention Center. “That coating can wall off the device from capillaries and prevent it from sensing chemical changes accurately, so it stops working after a few weeks or months.”
The sensors are smaller than a grain of rice and are made of a hydrogel scaffold that’s as flexible as a contact lens. With this new material, body cells and capillaries may grow into the sensor’s porous structure without triggering the undesirable immune response.
The sensors will analyze body oxygen, carbon dioxide, glucose or lactate, and other significant indicator to ones health. The collected information will be wirelessly transferred to a computer of a cellphone app.
This device is currently seeking U.S. Food & Drug Administration approval. However, in Europe the device has been approved for marketing under Profusa brand, and now being used to report tissue oxygen levels in patients under treatment for peripheral artery disease, which affects millions of people worldwide. The disease reduces the flow of oxygenated blood in arms and legs, in some cases leading to amputation. The device is being used to help prevent amputations by informing physicians about declining oxygen levels in a patient’s limbs.
Profusa is also starting a clinical trial with the University of California, San Francisco sponsored by the National Heart, Lung, and Blood Institute to use the sensors to track oxygen levels in patients with chronic foot wounds.
In addition, Profusa has been working toward perfecting sensors that can track additional analytes that physicians normally evaluate via standard blood tests. The idea is to inject a single sensor that detects multiple body chemistries at the same time. “The sensors would provide a continuous record of your analytes relative to your personal baseline,” Wisniewski explains.
Wisniewski says that the military is also interested in the device, and has provided support since the company’s inception. They envision using the sensors to evaluate the health of soldiers during deployment or to indicate which wounded soldiers to treat first on the battlefield. And today, Wisniewski is reporting preliminary results showing that tracking the rise and fall of oxygen levels around muscle with these sensors produces an “oxygen signature” that may reveal a person’s fitness level. That information could help the military determine the best training regimen for each soldier or could help athletes find the most effective exercises to build muscle or endurance.